Antifouling Properties

One of the primary approaches investigated at NDSU to create non-toxic, environmentally friendly antifouling coatings is to chemically attach or “tether” organic biocides into a coating matrix to deter settlement and or growth of marine fouling organisms. This strategy relies on the direct contact between the targeted fouling organisms and the coating surface to impart and antifouling character. A variety of high-throughput screening assays have been developed to rapidly characterize coatings based on this non-leaching, contact-active approach.
Bacterial Biofilm Growth
The primary workhorse of the high-throughput screening workflow for antifouling characterization is based on the utilization of marine bacteria. In particular, the marine bacteria employed are allowed to attach and colonize the coating surfaces for the appropriate period of time needed to generate a biofilm (i.e., 24-48 hours). One of the defining characteristics of bacterial biofilm formation is the generation of an extracellular polymeric substance (EPS). The EPS is predominantly composed on polysaccharides and protein and serves as effective matrix to not only adhere bacteria to surfaces or interfaces, but protect them from environmental adversity (i.e., predation, antifouling agents etc.).
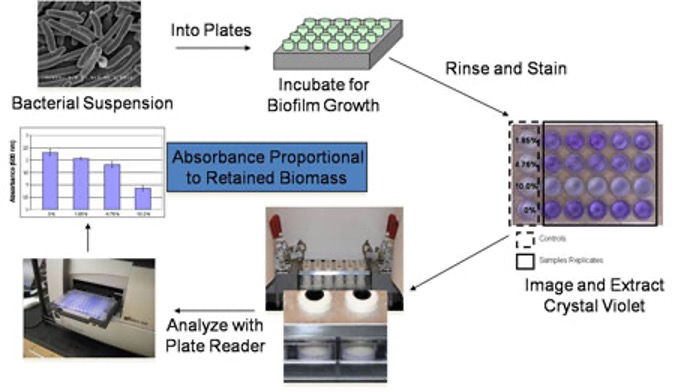
Marine bacteria are suspended in artificial sea water (ASW), supplemented with nutrients, and dispensed into 24-well plates containing cured experimental coating films. The plates are then incubated at the appropriate temperature and duration required to facilitate attachment and subsequent biofilm growth. Coating plates are then rinsed with ASW to remove loosely bound or planktonic growth, dried at ambient conditions, and stained with the biomass indicator dye, crystal violet. The crystal violet stained biofilms (purple color) are extracted with acetic acid and the resulting eluates are measured for absorbance at 600nm. The absorbance values obtained are directly proportional to the amount of biofilm growth retained on the coating surfaces.
Algal Biofilm Growth
Cultures of marine fouling microalgae are maintained year-round at NDSU for use in accelerated testing studies. The rapid testing assays employing microalgae are utilized in conjunction with bacteria to assess the antifouling properties of coating arrays for broad spectrum activity. Microalgae also live in multispecies biofilms, often with bacteria, which constitute ‘slime’ layers that facilitate adherence to surfaces and protection from the environment.
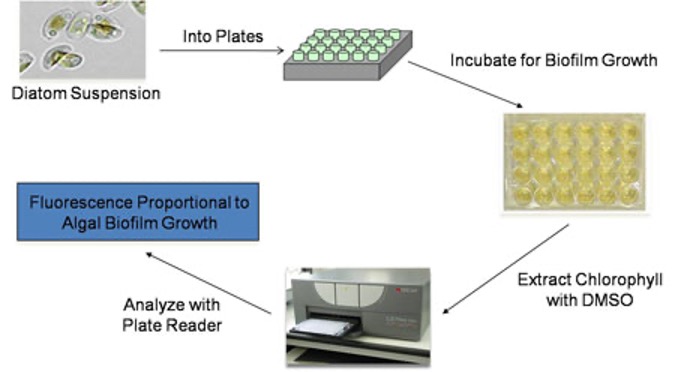
Microalgae are suspended in ASW, supplemented with nutrients, and dispensed into 24-well plates containing cured experimental coating films. The plates are then incubated at the appropriate temperature, duration and lighting conditions to facilitate attachment and subsequent biofilm growth. The ASW growth medium is removed and the plates are immediately treated with dimethyl sulfoxide (DMSO) to extract chlorophyll. The resulting eluates are then measured for chlorophyll fluorescence (Excitation: 360 nm; Emission: 670 nm). The fluorescence values obtained are directly proportional to microalgae biofilm growth cultured on the coating surfaces.


