Sanku Mallik, PhD

Professor
35B Sudro Hall
Phone (701) 231-7888
Fax (701) 231-8333
Education
Post doctoral (Bio-Organic Chemistry) 1993-1994
California Institute of Technology
PhD (Organic Chemistry) 1992
Case Western Reserve University
BS (Chemistry) 1987
Indian Institute of Technology
Kharagpur, India
Positions and Honors
- 2022, 2023 - Hogoboom Endowed Professorship
- 2017 - Waldron Award for Excellence in Research
- 2012 - Professor, Pharmaceutical Sciences, North Dakota State University
- 2006 - Associate Professor, Pharmaceutical Sciences, North Dakota State University
- 2005 - 2006 Associate Professor, Dept. of Chemistry, University of Central Florida
- 2002 - 2005 Associate Professor, Dept. of Chemistry, North Dakota State University
- 1998 - 2001 Assistant Professor, Dept. of Chemistry, North Dakota State University
- 1995 - 1997 Assistant Professor, Dept. of Chemistry, University of North Dakota
- 1994 - 1995 Senior Research Fellow, Division of Chemistry & Chemical Engineering, California Institute of Technology
- 1992 - 1994 Post doctoral Research Associate, Division of Chemistry & Chemical Engineering, California Institute of Technology. (Advanced Research Associateship, American Heart Association.)
- 1997 - CAREER development award, National Science Foundation.
Research
(1) Design of Isozyme-Selective Inhibitors for Matrix Metalloprotienase-9:
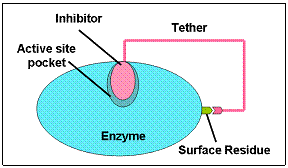 Over-expression of matrix metalloproteinases has been associated with a variety of human diseases, e.g., arthritis, cancer, cardiovascular diseases etc. Amongst the MMPs, MMP-2 and -9 have been found to overexpress in tumors of breast, prostrate, pancreas and ovary. In addition, these two enzymes are involved in angiogenesis, invasion and metastasis of tumors and in the immune response to cancer. Most of the reported inhibitors for MMPs are broad-spectrum inhibitors and have severe side effects. In contrast to the broad-spectrum inhibitors, the selective inhibitors are very promising as anti-metastatic agents.
Over-expression of matrix metalloproteinases has been associated with a variety of human diseases, e.g., arthritis, cancer, cardiovascular diseases etc. Amongst the MMPs, MMP-2 and -9 have been found to overexpress in tumors of breast, prostrate, pancreas and ovary. In addition, these two enzymes are involved in angiogenesis, invasion and metastasis of tumors and in the immune response to cancer. Most of the reported inhibitors for MMPs are broad-spectrum inhibitors and have severe side effects. In contrast to the broad-spectrum inhibitors, the selective inhibitors are very promising as anti-metastatic agents.
In collaboration with Prof. D. K. Srivastava (Biochemistry, NDSU), we have recently developed a highly-promising strategy for inhibitor design, called two-prong approach. In this approach, one part of the inhibitor binds to the active site region and the other part binds to the surface-exposed histidine residues.Since the surface-exposed histidine patches are not conserved among MMP isozymes, our contemplated two-prong inhibitor design approach will produce isozyme-specific inhibitors for MMP-9. The specific aims of this proposal are summarized below.
(2) Fabrication of hybrid liposomes with triple-helical collagen peptides for targeting to the matrix metalloproteinase-9:
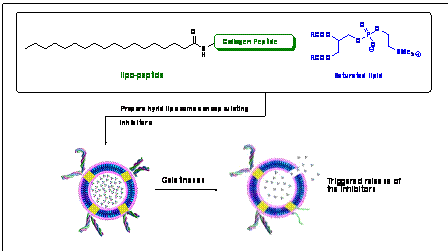 In collaboration with Prof. D. K. Srivastava ( North Dakota State University), this project aims to develop novel, triggered, liposomal drug delivery methods activated by an MMP-9. Hybrid, liposomes (incorporating lipids and lipo-peptides) are prepared encapsulating an inhibitor for the enzyme. The non-polymerized lipids present a peptide sequence on the liposome surface to be recognized and subsequently cleaved by the enzyme. The peptide cleavage will destabilize the non-polymerized domains of the liposomes, resulting in the formation of holes and (uncorking) of the liposomes. The encapsulated inhibitor will be released rapidly from the liposomes, inhibiting MMP-9. The peptide sequence can be suitably altered when inhibition of another MMP is necessary. This project combines the advantages of targeting and triggered release of unpolymerized liposomes with stability and rapid release property of hybrid liposomes to deliver drugs to a chosen MMP.
In collaboration with Prof. D. K. Srivastava ( North Dakota State University), this project aims to develop novel, triggered, liposomal drug delivery methods activated by an MMP-9. Hybrid, liposomes (incorporating lipids and lipo-peptides) are prepared encapsulating an inhibitor for the enzyme. The non-polymerized lipids present a peptide sequence on the liposome surface to be recognized and subsequently cleaved by the enzyme. The peptide cleavage will destabilize the non-polymerized domains of the liposomes, resulting in the formation of holes and (uncorking) of the liposomes. The encapsulated inhibitor will be released rapidly from the liposomes, inhibiting MMP-9. The peptide sequence can be suitably altered when inhibition of another MMP is necessary. This project combines the advantages of targeting and triggered release of unpolymerized liposomes with stability and rapid release property of hybrid liposomes to deliver drugs to a chosen MMP.
(3) Development of Chemical Receptors for Proteins Based on Polymerized Liposomes:
This project aims to fabricate highly selective, robust, chemical receptors for proteins employing polymerizable mixed liposomes. Nature uses antibodies to bind 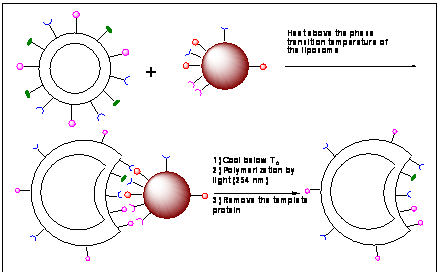 to a protein strongly and selectively. The goal of this project is to develop effective (synthetic antibodies). We prepare liposomes with polymerizable neutral lipids (zwitter ionic) as the major constituent. Other component lipids are metal-chelating and with charged head groups. After fabrication, these liposomes (in the unpolymerized state, above the gel-transition temperature) will be allowed to interact with the template protein. The metal ions on liposome surface will orient complementary to the pattern of surface-exposed histidines and/or acidic residues (Asp/Glu) of the enzyme. Quaternary ammonium headgroups on the liposomes will be positioned by acidic amino acid residues (Asp, Glu) on the protein surface. Amino acid side chains of the template protein capable of forming hydrogen bonds (e.g., Ser, Thr, Lys, Asn, Gln and Arg) will interact with the primary amine moieties on the liposome. The result of this equilibration step is the creation of a pattern of metal ions, charges and hydrogen bonding sites on the liposome complementary to the surface pattern exhibited by the protein of choice. We polymerize the resultant liposomes to prepare selective receptors for the protein of choice.
to a protein strongly and selectively. The goal of this project is to develop effective (synthetic antibodies). We prepare liposomes with polymerizable neutral lipids (zwitter ionic) as the major constituent. Other component lipids are metal-chelating and with charged head groups. After fabrication, these liposomes (in the unpolymerized state, above the gel-transition temperature) will be allowed to interact with the template protein. The metal ions on liposome surface will orient complementary to the pattern of surface-exposed histidines and/or acidic residues (Asp/Glu) of the enzyme. Quaternary ammonium headgroups on the liposomes will be positioned by acidic amino acid residues (Asp, Glu) on the protein surface. Amino acid side chains of the template protein capable of forming hydrogen bonds (e.g., Ser, Thr, Lys, Asn, Gln and Arg) will interact with the primary amine moieties on the liposome. The result of this equilibration step is the creation of a pattern of metal ions, charges and hydrogen bonding sites on the liposome complementary to the surface pattern exhibited by the protein of choice. We polymerize the resultant liposomes to prepare selective receptors for the protein of choice.
Publications
Synthesis of barbiturate-based inhibitors for Methionine Aminopeptidase-1. Haldar, M. K.; Scott, M. D.; Sule, N.; Srivastava, D. K.; Mallik, S. Bioorg. Med. Chem. Lett. 2008, 18, 2373-2376.
Novel bis-(arylsulfonamide) hydroxamate-based selective MMP inhibitors. Subramaniam, R.; Haldar, M. K.; Tobwala, S.; Ganguli, B.; Srivastava, D. K.;Mallik, S. Bioorg. Med. Chem. Lett. 2008, 18, 3333 - 3337.
Mechanistic studies on the triggered release of liposomal contents by matrix metalloproteinase-9. Elegbede, A. I.; Banerjee, J.; Hanson, A. A.; Tobwala, S.; Ganguli, B.; Wang, R.; Lu, X.; Srivastava, D. K.; Mallik, S. J. Am. Chem. Soc., 2008, 130, 10633-10642.
Unraveling the conformational states and microscopic environment of Carbonic Anhydrase I via a newly designed fluorophore. Manokaran, S.; Bortlund, J.; Kooren, J.; Banerjee, J.; Mallik, S.; Srivastava, D. K. Biochemistry, submitted.
A matrix metalloproteinase assisted triggered release of liposomal contents. Sarkar, N.; Banerjee, J.; Hanson, A. A.; Elegbede, A. I.; Rosendahl, T.; Krueger, A. B.; Banerjee, A. L.; Tobwala, S.; Wang, R.; Lu, X.; Mallik, S.; Srivastava, D. K. Bioconjugate Chem. 2008, 19, 57-64.
Energetic rationale for an unexpected and abrupt reversal of guanidine hydrochloride induced unfolding of peptide deformylase. Berg, A. K.; Manokaran, S.; Eiler, D.; Koorean, J.; Mallik, S.; Srivastava, D. K. Prot. Science 2008, 17, 11-15.
Structural Basis of Charge Discrimination in the Binding of Inhibitors to Human Carbonic Anhydrases I and II. Srivastava, D. K.; Jude, K. M.; Banerjee, A. L.; Haldar, M. K.; Mallik, S.; Christianson, D. W. J. Am. Chem. Soc. 2007, 129, 5528-5537.
Recognition of isozymes via lanthanide ion incorporated polymerized liposomes. Elegbede, A. I.; Haldar, M. K.; Manokaran, S.; Mallik, S.; Srivastava, D. K. J. Chem. Soc. Chem. Commun. 2007, 4495-4497.
Intrinsic selectivity in binding of MMP-7 to differently charged lipid membranes. Gangulay, B.; Elegbede, A. I.; Klocke, D. J.; Haldar, M. K.; Mallik, S.; Srivastava, D. K. FEBS Letters 2007, 581, 5723-5726.
New Fluorescent Probes for Carbonic Anhydrases. Banerjee, J.; Haldar, M. K.; Manokaran, S.; Mallik, S.; Srivastava, D. K. J. Chem. Soc. Chem. Commun. 2007, 2723-2725.
A Strategy for Designing "Multi-Prong" Enzyme Inhibitors by Incorporating Selective Ligands to the Liposomal Surface. Elegbede, A. I.; Haldar, M. K.; Manokaran, S.; Kooren, J.; Roy, B. C.; Mallik, S.; Srivastava, D. K. J. Chem. Soc. Chem. Commun. 2007, 3377-3379.
Surface-derivatized nanoceria with human carbonic anhydrase II inhibitors and fluorophores: a potential drug delivery device. Patil, S.; Reshetnikov, S.; Haldar, M. K.; Seal, S.; Mallik, S. J. Phys. Chem. C. 2007, 111, 8437-8442.
Artificial neural networks for qualitative and quantitative analysis of target proteins with polymerized liposomes vesicles. Santos, M.; Nadi, S.; Goicoechea, H. C.; Haldar, M. K.; Campiglia, A. D.; Mallik, S. Anal. Biochem 2007, 361, 109-119.
Partial-filling multiple-injection affinity capillary electrophoresis to estimate binding constants of receptors to ligands. Zavaleta, J.; Chinchilla, D.; Ramirez, A.; Pao, A.; Martinez, K.; Nilapwar, S. ; Ladbury, J. E. ; Mallik, S. ; Gomez, F. A. Talanta 2007, 71, 192-201.
Ultrahigh resolution crystal structures of human carbonic anhydrase I and II complexed with "two-prong" inhibitors. Jude, K. M.; Banerjee, A. L.; Haldar, M. K.; Roy, B. C.; Mallik, S.; Srivastava, D. K.; Christianson, D. W. J. Am. Chem. Soc.2006, 128, 3011-3018.
Formulation of photo-cleavable liposomes and the mechanism of their content release. Chandra, B.; Mallik, S.; Srivastava, D. K. Org. Biomol. Chem.2006, 4, 1730-1740.
Partial-filling multiple-injection affinity capillary electrophoresis to estimate binding constants of receptors to ligands. Zavaleta, J.; Chinchilla, D.; Ramirez, A.; Pao, A.; Martinez, K.; Nilapwar, S. ; Ladbury, J. E. ; Mallik, S. ; Gomez, F. A. Talanta, in press.
Synthesis of polymerizable lipids for sensitizing Dy 3+ and protein detection. Haldar, M.; Santos, M.; Rax, M.; Mallik, S.; Campiglia, A. D. Bioconjugate Chemistry, in press.
Triggered release of liposomal contents by a matrix metalloproteinase. Sarkar, N. R.; Rosendahl, T.; Krueger, A. B.; Banerjee, A. L.; Mallik, S.; Srivastava, D. K. Biochemistry, submitted.
Isozyme selectivity in binding of a bifunctional ligand with tumerogenic carbonic anhydrase XII in preference to carbonic anhydrase II. Banerjee, A. L.; Ganguly, B.; Haldar, M. K.; Buckle, M.; Roy, B. C.; Mallik, S.; Srivastava, D. K. J. Med. Chem., submitted.
Blocking the accessibility of the active site pocket of matrix metalloproteinase-9 by designing multiprong surface-binding groups. Banerjee, A. L.; Haldar, M. K.; Tolwala, S.; Swanson, M.; Roy, B. C.; Mallik, S.; Srivastava, D. K. J. Chem. Soc. Chem. Commun. 2005, 2549-2551.
Design of photo-cleavable lipids and their applications in liposomal "uncorking". Chandra, B.; Mallik, S.; Srivastava, D. K. J. Chem. Soc. Chem. Commun. 2005, 3021-3023.
Optimization of conditions for flow-through partial-filling affinity capillary electrophoresis to estimate binding constants of ligands to receptors. Brown, A.; Desharnais, R.; Roy, B.C.; Malik, S.; Gomez, F. A. Anal. Chim. Acta2005, 540, 403-410.
Spacer based selectivity in the binding of "two-prong" ligands to recombinant human carbonic anhydrase-I. Banerjee, A. L.; Eiler, D.; Roy, B. C.; Jia, X.; Haldar, M. K.; Mallik, S.; Srivastava, D. K. Biochemistry2005, 44, 3211-3224.
Solid-phase synthesis of polymerizable, lanthanide chelating lipids for protein detection. Nadi, S.; Santos, M.; Haldar, M. K.; Roy, B. C.; Mallik, S.; Campiglia, A. D. Inorganic Chemistry2005, 44, 2234-2244.
Evaluation of two lanthanide complexes for qualitative and quantitative analysis of target proteins via partial least squares analysis. Goicoechea, H.; Roy, B. C.; Santos, M.; Campiglia, A. D.; Mallik, S. Anal. Biochem.2005, 336, 64-74.
Triggered release of liposomal contents by matrix metalloproteinase-9. Sarkar, N. R.; Rosendahl, T.; Krueger, A. B.; Banerjee, A. L.; Mallik, S.; Srivastava, D. K. J. Chem. Soc. Chem. Commun. 2005, 999-1001.
Molecular basis for the origin of differential spectral and binding profiles of dansylamide with human carbonic anhydrase I and II. Banerjee, A. L.; Tobwala, S.; Srivastava, D. K.; Mallik, S. Biochemistry2005, 44, 3673-3682.
Two-prong inhibitors for human carbonic anhydrase II. Roy, B. C.; Banerjee, A. L.; Kloche, D. L.; Swanson, M.; Jia, X.; Haldar, M. K.; Mallik, S.; Srivastava, D. K. J. Am. Chem. Soc.2004, 126, 13206-13207.
Purification of recombinant human carbonic anhydrase-II without incorporating histidine tags. Banerjee, A. L.; Swanswon, M.; Mallik, S.; Srivastava, D. K. Protein Expr. Purif. 2004, 37, 450-454.
Protein surface-assisted enhancement of the binding affinity of an inhibitor for recombinant human carbonic anhydrase-II. Banerjee, A. L.; Swanson, M.; Roy, B. C.; Jia, X.; Haldar, M. K.; Mallik, S.; Srivastava, D. K. J. Am. Chem. Soc., 2004, 126, 10875-10883.
An investigation on the analytical potential of polymerized liposomes bound to lanthanide ions for protein analysis. Santos, M.; Roy, B. C.; Goicoechea, H.; Campiglia, A. D.; Mallik, S. J. Am. Chem. Soc., 2004, 126, 10738-10745.
Conjugation of surface binding groups to poor inhibitors: a strategy to improve inhibitor efficiency. Roy, B. C.; Rodendahl, T.; Hegge, R.; Peterson, R.; Mallik, S.; Srivastava, D. K. J. Chem. Soc. Chem. Commun., 2003, 2328 - 2329.
Synthesis of metal-chelating lipids to sensitize lanthanide ions. Roy, B. C.; Santos, M.; Mallik, S.; Campiglia, A. D. J. Org. Chem.2003, 68, 3999 - 4007.
Synthesis of new, pyrene-containing, metal-chelating lipids and sensing of cupric ions. Roy, B. C.; Chandra, B.; Hromas, D.; Mallik, S. Org. Lett. 2003, 5, 11-14.
Thermodynamic studies on the recognition of flexible peptides by transition metal complexes. Sun, S.; Fazal, A. Md.; Roy, B. C.; Mallik, S. Inorg. Chem.2002, 41, 1584-1590.
Surface recognition of a protein using designed transition metal complexes. Fazal, A. Md.; Roy, B. C.; Sun, S.; Mallik, S.; Rodgers, K. R. J. Am. Chem. Soc. 2001, 123, 6283 - 6290.
Synthesis of conjugated, diacetylene, metal-chelating momoners for polymerizable monolayer assemblies. Roy, B. C.; Mallik, S. Org. Lett. 2001, 3, 1877 - 1879.
Polymerized fluorescent liposomes incorporating lanthanide ions. Roy, B. C.; Fazal, A. Md.; Arruda, A.; Mallik, S.; Campiglia, A. D. Org. Lett . 2000, 2 , 3067 - 3070.
Synthesis and fluorescence properties of new, fluorescent, polymerizable, metal-chelating lipids. Roy, B. C.; Peterson, R.; Mallik, S.; Campiglia, A. D., J. Org. Chem. 2000 , 65 , 3644-3651.
Recognition of flexible peptides in water by transition metal complexes. Sun, S.; Fazal, A. Md.; Roy, B. C.; Mallik, S. Org. Lett . 2000, 2 , 911-915.
Selective recognition of carbonic anhydrase using transition metal complexes. Roy, B. C.; Fazal, A. Md.; Sun, S. J. Chem. Soc. Chem. Commun. 2000 , 547-548.
C. Patents
Mallik, S .; Sarkar, N. R.;Srivastava, D. K.; Krueger, A. (2005); Controlled release liposomes and methods of use. U. S. Patent Application filed on September 15, 2005 by Mueting & Raasch, Minneapolis.
Mallik, S. ; Roy, B. C.; Srivastava, D. K. (2004); Methods and materials for enhancing the effects of protein modulators. Provisional Patent application filed on July 8, 2004 by Regalsky & Weyland, New York (Docket # 047-00050).
Mallik, S. ; Roy, B. C.; Srivastava, D. K. (2004); Methods and materials for enhancing the effects of protein modulators. Full Patent application filed on August 3, 2004 by Regalsky & Weyland, New York (Docket # # 047-00052).


