Take Home Message: An ongoing clubroot survey for over five years in various counties of North Dakota indicates a threat to the canola crop if proper attention is not given towards longer crop rotations (1 in 3 years). In addition, growers should consider growing an available clubroot resistant canola variety in endemic areas and follow proper equipment sanitation. Cleaning equipment thoroughly after working in a clubroot infected field is highly recommended since the primary mechanism of spread between fields is the movement of infested soil on farm equipment.
Survey Procedure: The survey involved three components 1. visual survey, 2. soil sampling and 3. molecular quantification of resting spores of the clubroot pathogen.
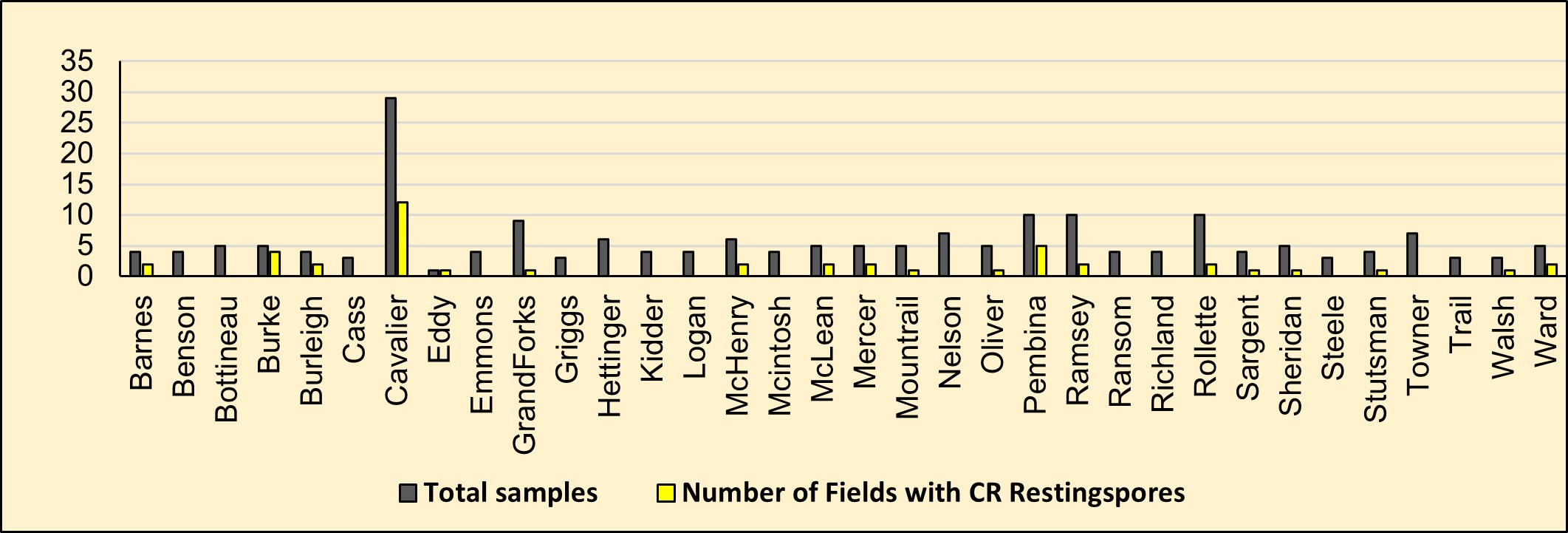
Components 1&2. Visual survey and soil sampling: Clubroot scouting was done visually by inspecting canola crop roots. The disease survey was conducted in over 40 counties in North Dakota. In each county, one field in every 5000 acres was targeted for scouting. Soil samples were collected from fields with an intent to know the pH of the soil and to determine the number of resting spores per gram of soil. In all, a minimum of 3-10 fields per county were targeted for scouting.
The survey was done in two phases.
1st phase: at flowering (10% of flowering onwards)
In the growing season, plants were sampled from distinct stunted patches or prematurely senescing plants in the field. Patches visible from the edge of the field were checked by digging and observing the roots for symptoms of clubroot and soil samples were collected from those areas.
2nd phase: after swathing
Scouting at swathing was based on the methodology followed in Canada by the Alberta Agricultural and Rural Development (AARD) for their annual clubroot disease survey. Reports of AARD indicated that the incidence of clubroot is increased in the field entrances. Hence, the survey was done from the main entrances/approaches in each field. The survey group walked a “W” pattern stopping at 5 spots and uprooting 10 consecutive stems from the ground at each spot. Each sampling point was separated by 100 meters or 328 feet. In all, roots of 50 stems were evaluated for the presence of clubroot and incidence was noted. Excess soil was removed. Roots were visually examined for the presence of galls. At sample sites where infection was observed or suspected, root specimens with galls, along with soil, were double bagged and labeled with the field location. Infected roots and soil samples from all the fields surveyed were collected and a representative sample was submitted to Dr. Zhaohui Liu’s laboratory for molecular quantification of resting spores per gram of soil and another half-pound of soil to the NDSU Soil Testing Laboratory for pH determination.
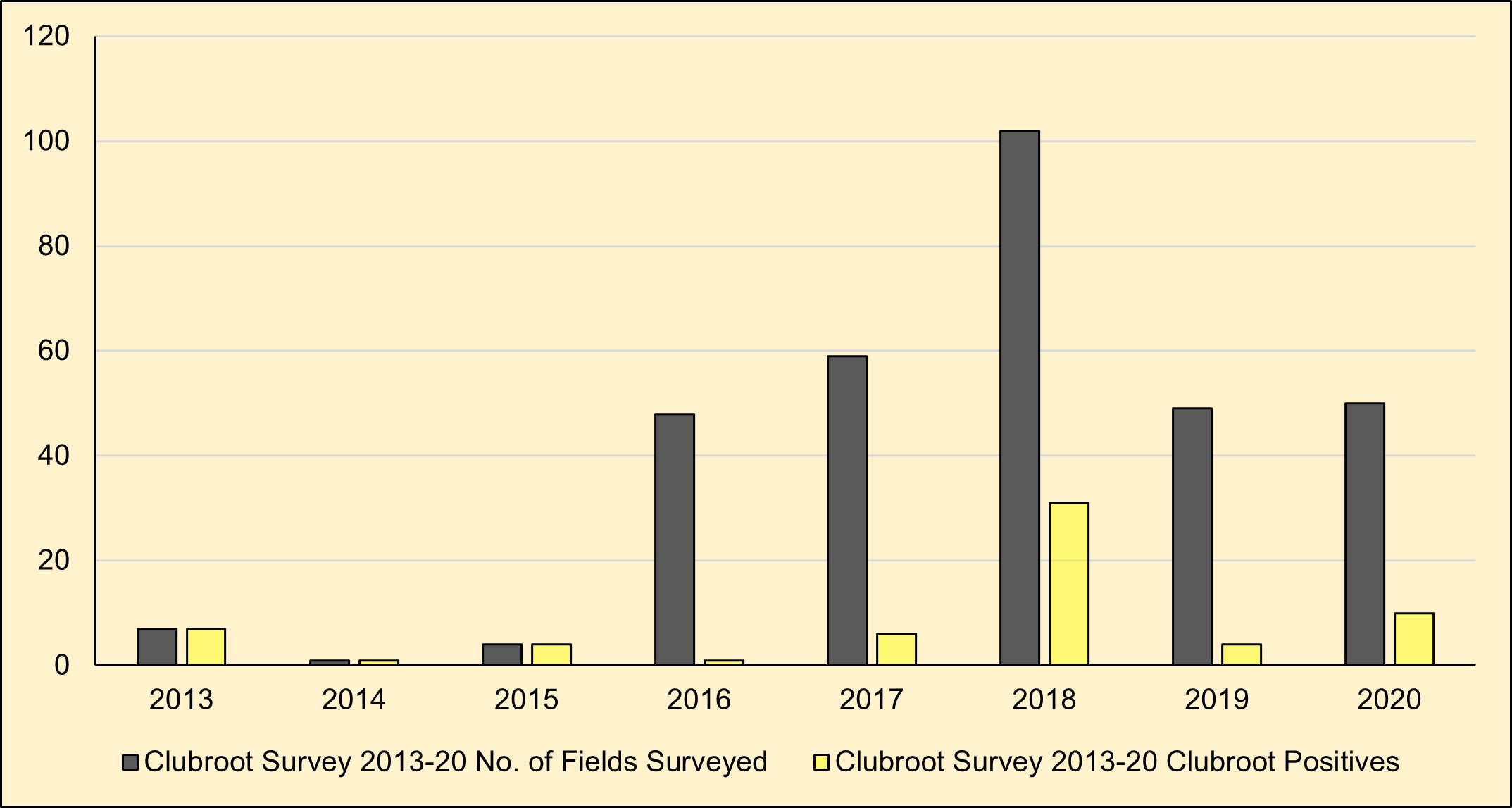
Results: Over 40 counties in North Dakota were surveyed in 2020 for visual symptoms of galls on canola roots. Clubroot galls on canola roots have only been found in Cavalier County, in 10 out of 50 canola fields surveyed (Figure 1).
Figure 1: Fields surveyed from 2013 to 2020 for prevalence of clubroot in Cavalier County, North Dakota.