Clubroot on Canola: Survey & Quantification of Resting Spores of Plasmodiophora brassicae from Field Collected Soil Samples in North Dakota
Principle Investigator: Venkat Chapara
Collaborators: Dante Marino, Ibukunoluwa Bankole, Amanda Arens, Travis J. Prochaska, Audrey Kalil, Jingwei Guo, Gongjun Shi, Zhaohui Liu, Luis del Rio, Kishore Chittem and Anitha Chirumamilla
Take Home Message: An ongoing clubroot survey for over six years in various counties of North Dakota indicates a threat to the canola crop if proper attention is not given towards longer crop rotations (one in three years). In addition, growers should consider growing an available clubroot resistant canola variety in endemic areas and following proper equipment sanitation. Cleaning equipment thoroughly after working in a clubroot infected field is highly recommended since the primary mechanism of spread between fields is the movement of infested soil on farm equipment.
Survey Procedure:
The survey involved three components: 1. visual survey, 2. soil sampling, and 3. molecular quantification of resting spores of the clubroot pathogen.
Components 1&2. Visual survey and soil sampling: A clubroot disease survey was conducted in fifty counties of North Dakota to determine prevalence of Plasmodiophora brassicae. The visual survey was done by inspecting canola crop roots. One field in every 5,000 acres was targeted for scouting in each county. Soil samples were collected from the visited fields to determine the pH of the soil and the number of resting spores per gram of soil. A minimum of three to ten fields per county were targeted for scouting.
The survey was done in two phases.
1st phase: at flowering (10% of flowering onwards)
Plants were sampled from distinct stunted patches or prematurely senescing plants in the field during the growing season. Patches visible from the edge of the field were checked by digging and observing the roots for symptoms of clubroot and soil samples were collected from those spots.
2nd phase: after swathing
Scouting at swathing was based on the methodology followed in Canada by the Alberta Agricultural and Rural Development (AARD) for their annual clubroot disease survey. Reports of AARD indicated that the probability of finding clubroot was higher if scouted at the field entrances. Hence, the survey was done starting from the main entrances/approaches in each field. The survey group walked in a “W” pattern stopping at five spots and uprooting ten consecutive stems from the ground at each spot. Each sampling point was separated by 100 meters or 328 feet. Roots of fifty stems were evaluated for the presence of clubroot and incidence. After removing excess soil, roots were visually examined for the presence of galls. At sample sites where infection was observed or suspected, root specimens with galls, along with soil, were double bagged and labeled with the field location. Infected roots and soil samples from all the fields surveyed were collected and a representative sample was submitted to Dr. Zhaohui Liu’s laboratory for molecular quantification of resting spores per gram of soil. An additional half-pound of soil was sent to the NDSU Soil Testing Laboratory for pH determination.
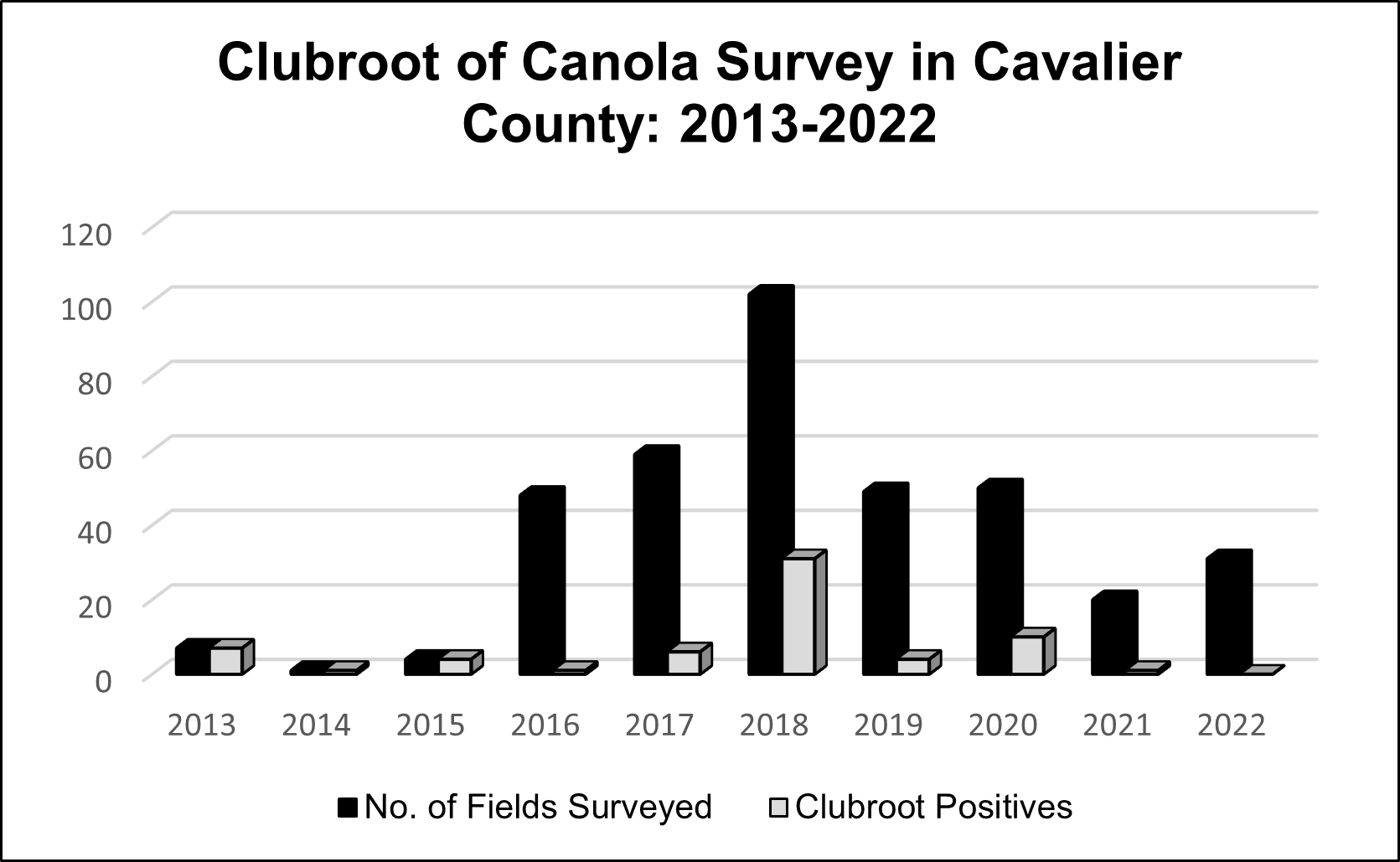
Results: During the two-year study of clubroot survey in North Dakota, only one field out of the fifty counties had canola roots with galls that were infused by clubroot pathogen (Figure 1). There is a declining trend in the number of clubroot infected canola fields since 2019. The decline in clubroot could be attributed to the change in crop production practices by the growers such as implementing longer rotations and the use of clubroot resistant cultivars.
Figure 1: Fields surveyed from 2013 to 2022 for prevalence of clubroot in Cavalier County, North Dakota.
Component 3. Molecular detection of soil samples to quantify Plasmodiophora brassicae (the clubroot pathogen) resting spores:
Over 500 samples were collected in fifty counties of North Dakota and were submitted for resting spore quantification and pH determination.
The main objective of this procedure is to quantify resting spores of the clubroot pathogen from the soil and to determine the pH of the soil. The information will be useful for growers to decide on the suitable crop for the rotation and to be aware of the infection levels of clubroot pathogen in their fields.
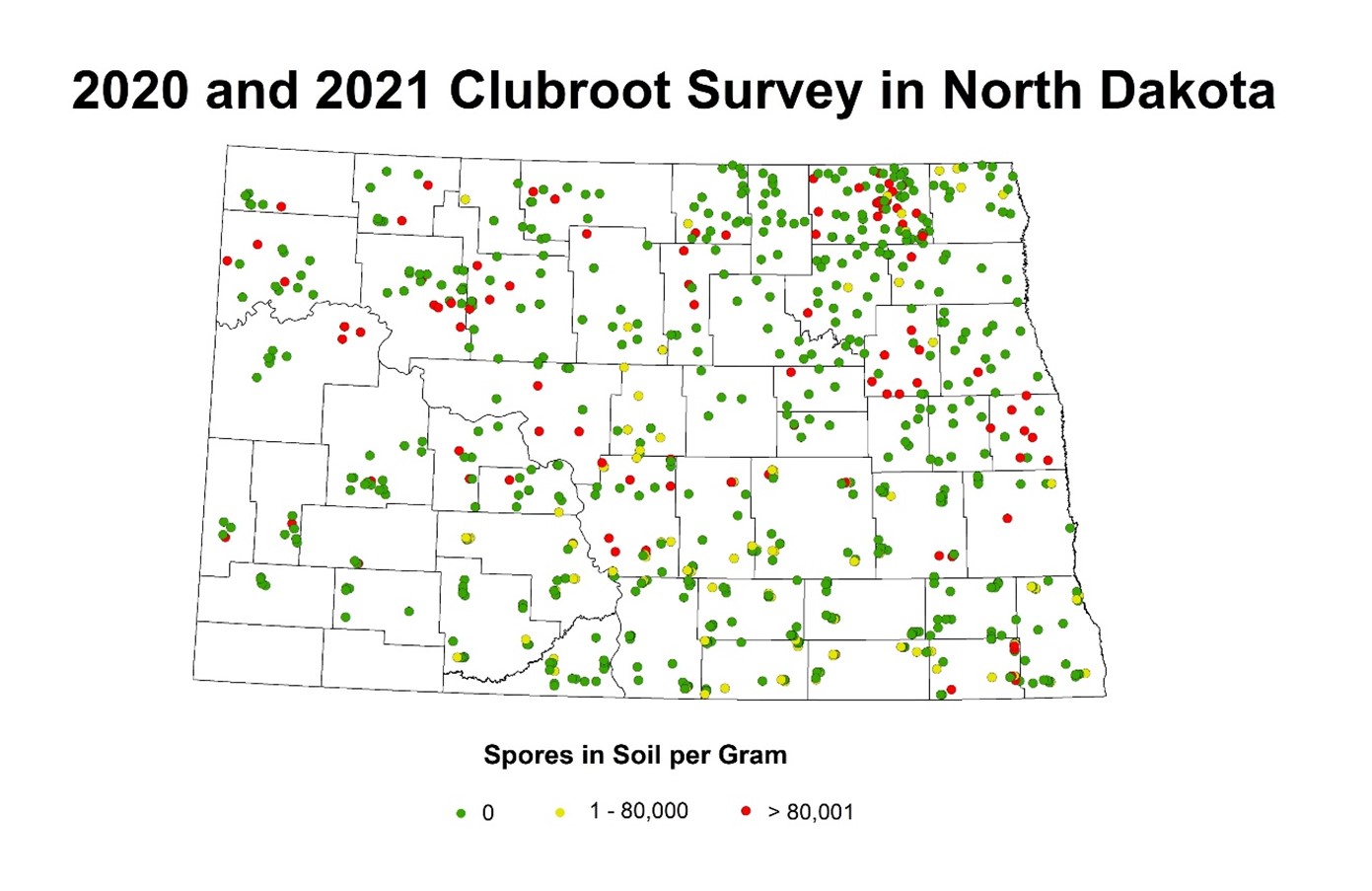
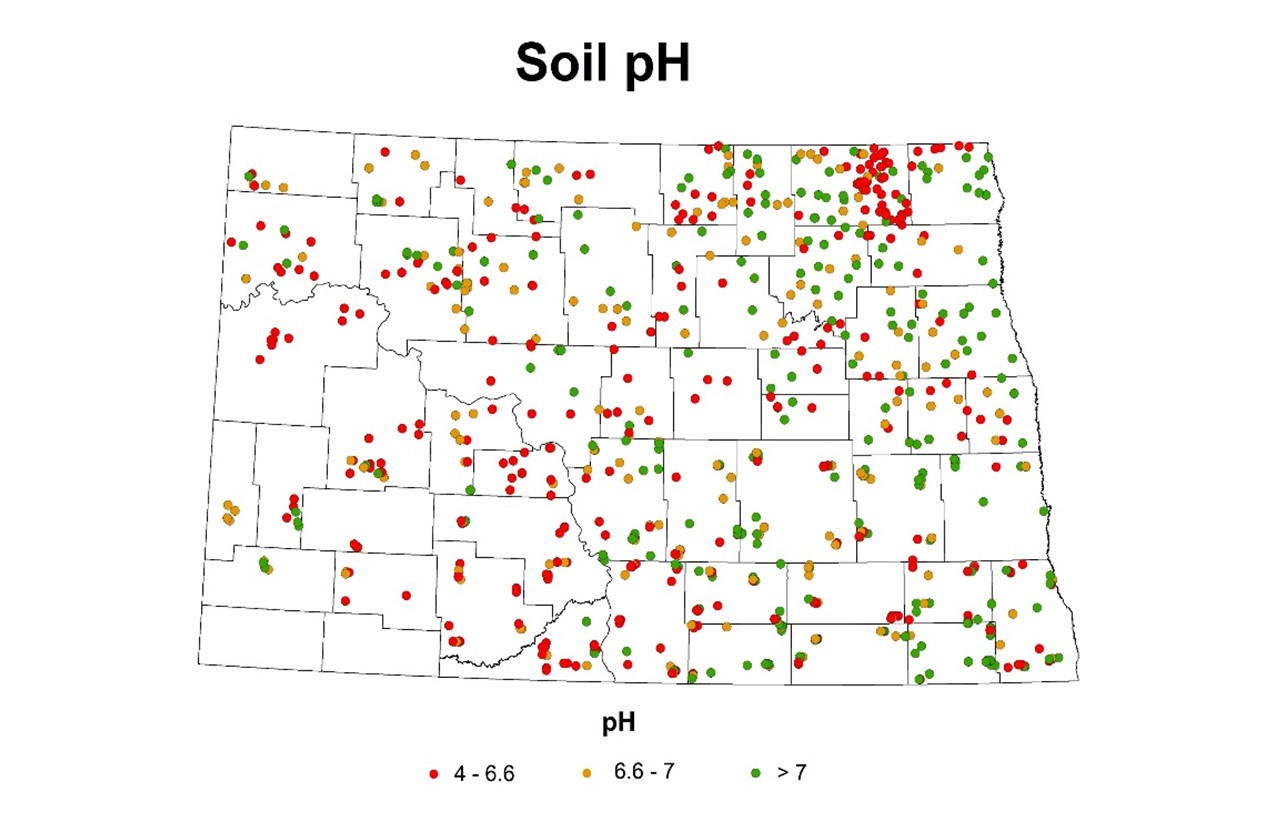
Results from molecular assays on soil samples: The molecular assays indicated that the clubroot pathogen resting spores have been found in 48 out of 50 counties (Figure 2) that were surveyed. Quantified resting spores of P. brassicae from those samples ranged from 500 to 40,000,000 spores per gram of soil (minimum detection limit of the assay being 10 resting spores/g of soil). Although there were no visible symptoms observed when the roots were uprooted in the surveyed fields, positives in the molecular soil quantification assay indicate either the resting spore population may not have reached required spores per gram of soil in acidic soils to show galls or the pH of the soil is basic (Figure 3). In general, clubroot infections are expressed on canola plants where soil resting spore population totals about 80,000 spores per gram of soil (Canadian Research). These results indicate that there is a need for continuous annual monitoring of clubroot spread in North Dakota.
Notice: Growers who are curious about the presence of clubroot/resting spores in their field(s) are encouraged to contact Dr. Venkat Chapara at the Langdon REC (701-256-2582), NDSU Cavalier County Extension Office (701-256-2560), or NDSU Extension (701-231-8363).
Figure 2: Map of counties in North Dakota indicating the presence and the number of Plasmodiophora brassicae (the clubroot pathogen) resting spores (detected by molecular assays) in the soil.
Note: The green dot indicates zero, yellow dot indicates range of 1-80,000 and the red dot indicates more than 80,000 resting spores per gram of soil.
Figure 3: Map of counties in North Dakota indicating pH ranges detected by soil assays in the soil samples during the two-year survey.
Acknowledgments: Funding from the Northern Canola Growers Association and the Northern canola research program (USDA). Thanks to all the product suppliers and the NDSU Soil Testing Lab. Special thanks to Dr. Knodel, and Dr. Honggang, and interns Jacob Kram (NDSU), Parker Rime, Brock Freer, Larissa Jennings and Iverson Peltier.