FHB-infected grain may contain fungus-produced toxic substances called mycotoxins. The most common mycotoxin associated with Fusarium-infected grain in the northern Great Plains is deoxynivalenol or DON (vomitoxin). This mycotoxin may cause vomiting and feed refusal in nonruminant animals, such as pigs.
The presence of this toxin may result in substantial price discounts at the market and even refusal to purchase if DON toxin levels are high. In the case of barley used by the malting and brewing industry, DON level requirements are very low, generally less than 0.5 part per million (ppm; milligrams/kilogram).
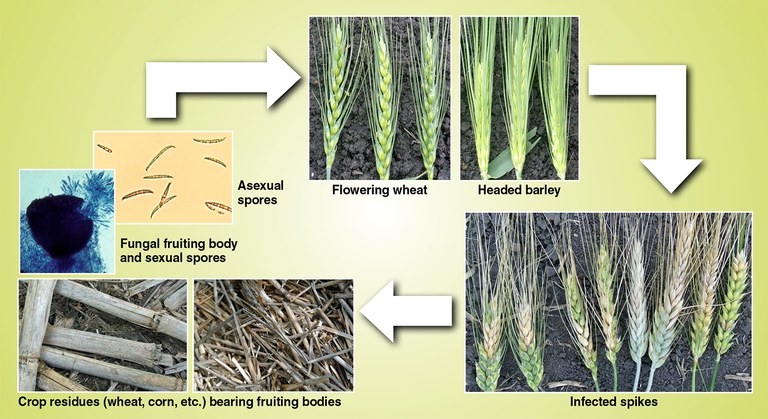
The presence of Fusarium-infected grain does not automatically mean mycotoxins are present. The occurrence, amount and kind of mycotoxins may depend on several factors, including environment, species of fungus present, severity of infection and the variety or kind of crop. Fusarium-infected grain may be tested for DON and other mycotoxins at properly equipped laboratories.
Contact a veterinarian or feed specialist for further information on safe livestock feeding levels. The risk of human exposure to DON ingestion is minimal under the Food and Drug Administration (FDA) guidelines (see table), but producers and elevator operators need to be aware that moldy grain can cause allergy and breathing problems. Producers and elevator operators should wear a good-quality dust mask when working around grain with high amounts of scab or other molds.
The FDA has established the following advisory levels for DON in food and feed:
- 1 ppm for finished grain products for human consumption
- No standard for raw grain going into milling process
- Cattle more than 4 months old: 10 ppm (providing grain at that level doesn’t exceed 50% of diet)
- Poultry: 10 ppm (providing grain at that level doesn’t exceed 50% of diet)
- Swine: 5 ppm (not to exceed 20 percent of ration)
- All other animals: 5 ppm (providing grains don’t exceed 40% of diet)
This publication was authored by Marcia P. McMullen, former NDSU Extension cereal pathologist; Shaobin Zhong; and Stephen Neate, former NDSU barley pathologist, 2008.
Revised Sept. 2018