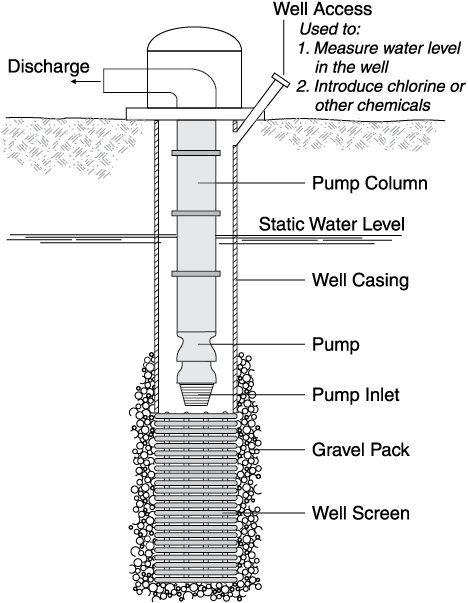
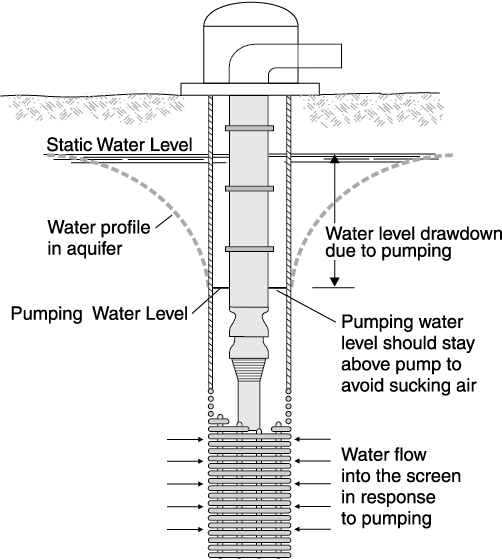
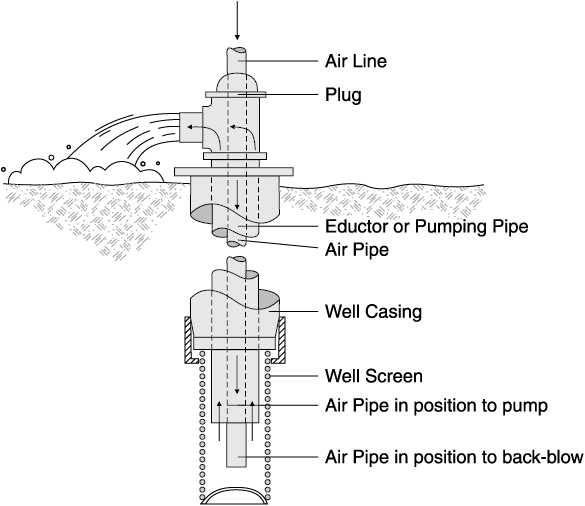
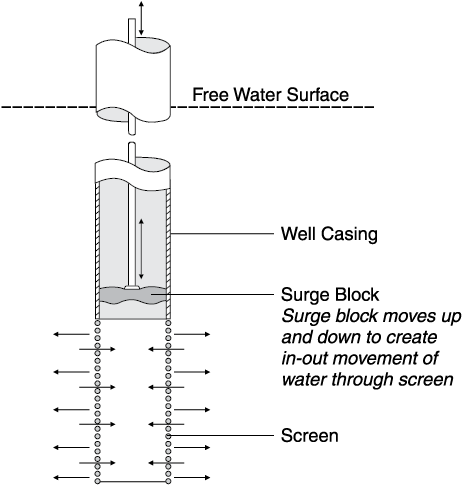
Chemically treating a well usually refers to using some form of strong acid to dissolve the mineral incrustation on the screen, casing and in the gravel pack or aquifer surrounding the screen. Often this is called “acidizing” a well. However, because of water chemistry, using a strong base might be better than acid in some cases.
Many types of acid can be used (see section on “Common Well Cleaning Acids”) for this purpose, but the well driller should remember that acid selection is site-specific and depends on the materials used to make the casing and screen, as well as water quality and aquifer materials.
Screens made from plastic, fiberglass and stainless steel are resistant to most strong acids and bases used for chemical treatment. Strong acids can damage screens made from perforated iron casing, galvanized iron cage-wound screens and steel screens.
Common Well-cleaning Acids
All chemicals to clean wells should be labeled for use in water wells.
The amount of chemical added to a well should be based on the quantity of water in the well. Often adding too much acid to a well may not help and actually can hinder the rehabilitation process. With severe mineral incrustation, doing the acidizing in two steps often is better.
Use the first batch of cleaning chemicals to dissolve some of the minerals, then pump them out and add the second batch to dissolve the remaining parts. Wear the proper safety equipment, as recommended by the chemical manufacturer, when handling well-cleaning acids. Equipment should include goggles, masks, rubber gloves and full clothing coverage. In addition, a supply of clean water for eyewash and rinsing spills should be available.
Muriatic Acid
Muriatic acid, a common product used for acidizing a well, is an industrial name for a hydrochloric acid solution with about 30 percent concentration, a very strong acid. It provides a fast chemical reaction to dissolve carbonate scales and incrustation. It is particularly effective against iron and manganese oxides but doesn’t remove biological buildup very effectively.
Although good for cleaning wells, hydrochloric acid can be dangerous to handle. Excessive amounts in the well can produce large amounts of toxic fumes. Inhaling these fumes can cause death. Use only the recommended amount of acid for the volume of water in the well. Only professionals with training and access to proper safety equipment should handle this acid.
Sulfamic Acid
This is a type of sulfuric acid and comes in a dry form. It is safer to use than muriatic acid but has a moderate chemical reaction, so it takes longer to dissolve carbonate scales and incrustations. It is not very effective against sulfate mineral deposits.
Sulfate is present in relatively large amounts in much of the groundwater in North Dakota. Sulfamic acid doesn’t produce harmful fumes and is not very corrosive. It isn’t very effective at removing biological buildup.
Phosphoric Acid
Phosphoric acid is a mild acid that contains phosphorus, which if discharged into wetlands or water bodies, can increase algae buildup. It is less corrosive to metal than muriatic acid. It is somewhat effective in dissolving iron and manganese oxides but is not very effective against biological buildup in the well.
Glycolic Acid
Glycolic acid, also known as hydroxyacetic acid, is effective against biological accumulations. It will disperse and help remove biofilms that build up on the screen, pump and casing. The chemical reaction is slow and creates no harmful fumes.
Acid Combinations
To remove biological products and mineral incrustations effectively at the same time, well drillers often use a combination of acids when rehabilitating a well. One combination is muriatic and glycolic acid, with each added to a volume of water at about the same percentage by weight or volume. More often, well drillers will use commercial products that are premixed acid combinations designed to address specific incrustation problems.